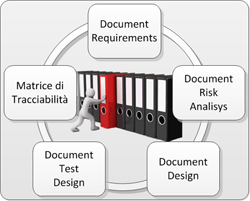
In all the phases of the project (design, development and validation), Novagem Solutions follows a specific path, with great care to the creation of the necessary documentation. In the first phase, it is written a TRS (Technical Requirements Specification), which helps to define a better problem comprehension. The second document is a Risk Analysis, which treats every aspect of solution feasibility in terms of security, sturdiness and fidelity of data. It is also really important to take care of things like the future scalability and the interfacing with standard systems and protocols.The next phase after the realization of the solution is the production of the Design Document, which declares how the subsystems have been addressed and implemented. Then there is the design of debug test protocols to verify the systems, also called Test Design phase. When the software is ready it is “frozen” and labeled with a specific internal release and then the Test Execution phase can occur. Last the Traceability Matrix is produced, which cover the entire path of verification and validazion.
|
|
The security and the traceability that imbue the biomedical market require an increasingly number of solution Part-11 compliant.
Part-11 identifies a series of guidelines for electronic data acquisition, storaging and handling, defined inside the Code of Federeal Regulations, wrote by the FDA (Food and Drug Administration).
Following the Part-11 criterias and provisions it is certified that electronic data will have the same value of papery ones. Data management processes are certified, starting from creation, to editing, storaging, data recovery and transmission. Corporations that offer products or services in the fields of food, medicine, pharmaceutical, biotechnology, biological research, biotech, healthcare and cosmetics need to respect those rules. Audit trail certifies the revision of a program, but also of data, allowing the management and the track keeping of historical transation concerning it. Each step of a process or a data is identified, since its creation to its completion, tracking every change made and providing the chance to do a roll back.
|